Unveiling the unheated gas in the above system, this exploration delves into the physical properties, behavior, and control strategies surrounding this enigmatic component. Its presence significantly influences system dynamics, demanding a thorough understanding of its characteristics and impact.
Unheated gases exhibit unique properties that govern their behavior within a system. Temperature plays a crucial role in determining their physical characteristics, affecting their volume, pressure, and energy levels. Various gases, such as nitrogen, helium, and argon, often remain unheated in specific system configurations.
Unheated Gas Properties

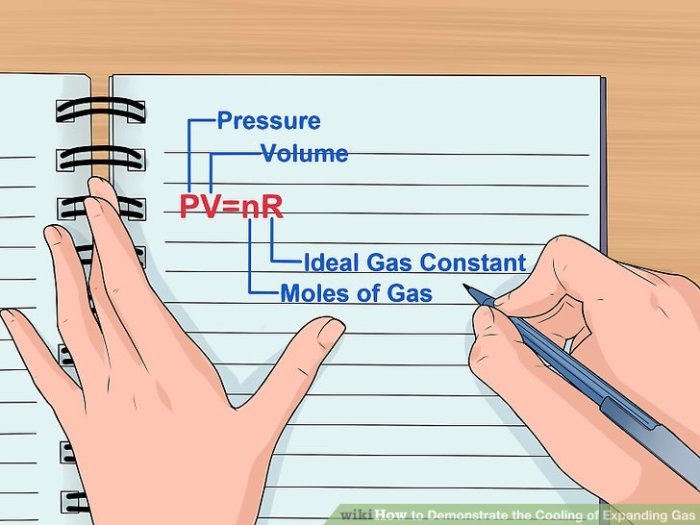
Unheated gas in a system refers to gases that remain at ambient temperature, without the application of external heat sources. These gases exhibit specific physical properties that govern their behavior and interactions within the system.The temperature of a gas plays a crucial role in determining its behavior.
According to the ideal gas law, the pressure, volume, and temperature of a gas are directly proportional. As temperature increases, the average kinetic energy of gas molecules increases, leading to an increase in pressure and volume. Conversely, when temperature decreases, the kinetic energy decreases, resulting in a decrease in pressure and volume.Examples
of gases that remain unheated in a system include air, nitrogen, and helium. These gases are commonly used in various industrial and scientific applications due to their inert nature and stability at ambient temperatures.
Gas Behavior in an Unheated System
In an unheated system, the gas remains at a constant temperature, exhibiting specific behaviors that impact the overall system dynamics and interact with other system components.
The unheated gas tends to expand and fill the available space within the system, exerting pressure on the system boundaries. This expansion is driven by the thermal energy of the gas molecules, which increases their kinetic energy and causes them to move more rapidly and collide with each other and the system walls.
Impact on System Dynamics
The unheated gas affects the system dynamics by introducing a force that opposes any external pressure applied to the system. This force, known as gas pressure, acts in all directions, pushing against the system boundaries and resisting any attempt to compress the gas.
The gas pressure also affects the flow of fluids within the system. When the gas pressure is high, it can restrict the flow of fluids, causing a decrease in flow rate. Conversely, when the gas pressure is low, it allows fluids to flow more easily, resulting in an increase in flow rate.
Impact on Other System Components, The unheated gas in the above system
The unheated gas can impact other system components by transferring heat through conduction, convection, or radiation. This heat transfer can affect the temperature and performance of these components.
For example, if the unheated gas is in contact with a solid surface, it can transfer heat to the surface, causing the surface temperature to rise. This heat transfer can affect the thermal properties of the solid, such as its thermal conductivity and specific heat capacity.
Measurement and Detection of Unheated Gas: The Unheated Gas In The Above System
The presence of unheated gas in a system can be detected using various measurement techniques. These techniques rely on specific principles and utilize sensors or devices designed for gas detection.
Gas Chromatography
Gas chromatography (GC) is a widely used technique for separating and analyzing gases. It involves passing the gas sample through a column packed with a stationary phase. Different gases in the sample will interact with the stationary phase to varying degrees, causing them to elute (come out of the column) at different times.
The elution times can be used to identify and quantify the gases present.
Mass Spectrometry
Mass spectrometry (MS) is a powerful technique for identifying and characterizing gases. It involves ionizing the gas sample and then separating the ions based on their mass-to-charge ratio. The resulting mass spectrum can be used to identify the gases present in the sample.
Infrared Spectroscopy
Infrared (IR) spectroscopy measures the absorption of infrared radiation by gases. Different gases absorb IR radiation at specific wavelengths, which can be used to identify and quantify the gases present. IR spectroscopy is particularly useful for detecting gases that have strong IR absorption bands, such as carbon dioxide and methane.
Thermal Conductivity Detectors
Thermal conductivity detectors (TCDs) measure the thermal conductivity of a gas sample. Different gases have different thermal conductivities, which can be used to identify and quantify the gases present. TCDs are often used in combination with GC to provide additional information about the gas sample.
Control and Management of Unheated Gas
Controlling and managing unheated gas is crucial for ensuring system safety and efficiency. Various strategies are employed to regulate the gas flow, pressure, and temperature within acceptable limits.
Control Methods
Control methods for unheated gas typically involve regulating flow rates, adjusting pressures, and maintaining desired temperatures. Common methods include:
- Flow Control Valves:These valves regulate the flow of gas by adjusting the opening or closing of a valve, thereby controlling the volume of gas passing through.
- Pressure Regulators:Pressure regulators maintain a constant gas pressure by sensing the downstream pressure and adjusting the flow of gas accordingly.
- Temperature Control Systems:These systems use sensors to monitor gas temperature and adjust the flow of gas or activate cooling/heating mechanisms to maintain the desired temperature.
Benefits and Limitations
Each control method offers specific benefits and limitations:
- Flow Control Valves:
- Benefits:Precise flow control, relatively simple to implement.
- Limitations:Can introduce pressure drops, potential for leaks.
- Pressure Regulators:
- Benefits:Maintain constant pressure, improve system stability.
- Limitations:May not be suitable for high-flow applications, can be sensitive to pressure fluctuations.
- Temperature Control Systems:
- Benefits:Prevent overheating or freezing of gas, ensure optimal system performance.
- Limitations:Can be complex and expensive to implement, require accurate temperature sensing.
Control Systems and Algorithms
Advanced control systems and algorithms are employed to optimize the control of unheated gas. These systems may use:
- Proportional-Integral-Derivative (PID) Controllers:These controllers adjust control actions based on the error between the desired and actual values.
- Fuzzy Logic Controllers:These controllers use fuzzy logic to make decisions based on imprecise or incomplete information.
- Model Predictive Control (MPC):This predictive control technique uses a model of the system to predict future behavior and optimize control actions.
Answers to Common Questions
What are the key properties of unheated gases?
Unheated gases exhibit low temperature, low energy levels, and relatively constant volume and pressure.
How does unheated gas affect system behavior?
Unheated gas can alter system dynamics by influencing pressure gradients, heat transfer, and flow patterns.
What methods are used to measure unheated gas?
Temperature sensors, pressure gauges, and gas analyzers are commonly used to detect and quantify unheated gas.
What control strategies can be employed for unheated gas?
Control strategies include temperature regulation, pressure control, and flow management to maintain desired gas properties.